The zinc ion (Zn2+) is a common ingredient of many over-the-counter multivitamin and mineral dietary supplements. Many enzymes incorporate Zn2+ as a co-factor and participate in various physiological activities, such as apoptosis, DNA synthesis, gene expression, immunity, and neurotransmission. The free intracellular Zn2+ concentration ([Zn2+]i) is maintained at a sub-nM level by Zn2+ transporters at the membranes of organelles and binding proteins. Our previous reports have shown that treating neurons with dopamine or Zn2+ elevates [Zn2+]i and causes cell death. Dopamine elevates [Zn2+]i with a change of hundreds of pM and activates the autophagic pathway via the D1 receptor-protein kinase A (PKA)-nitric oxide (NO) cascade in cultured cortical neurons. In addition, blocking this elevation in [Zn2+]i suppresses dopamine-induced autophagosome formation (Hung et al., 2017). These findings reveal that intracellular Zn2+ homeostasis is important for the activation of various signaling pathways responsible for neuronal survival.
In synaptic vesicles of glutamatergic neurons, a high concentration of Zn2+, 100-300 μM, is coreleased with glutamate upon stimulation, thereby elevating the local extracellular Zn2+ concentration ([Zn2+]e). Abundant extracellular Zn2+-chelating proteins are suggested to maintain [Zn2+]e at a low sub-nM level. Extracellularly, Zn2+ can bind to the N-methyl-D-aspartate (NMDA) receptor and regulate the kinetics of the conjugated ion channels; Zn2+ can also facilitate the aggregation of amyloid β (Aβ) and enhance neurodegeneration. Some Zn2+-related metal ionophores, such as PBT2, are under clinical trials to verify the importance of Zn2+ homeostasis in neurodegenerative diseases. However, most studies adopt a high concentration of Zn2+ (> 100 mM), and whether physiological levels of [Zn2+]e are sufficient to activate these physiological responses is not clear, as traditional biological approaches cannot accurately monitor [Zn2+]e in real time.
Over the past several years, with the collaboration of Dr. Yit-Tsong Chen (Department of Chemistry, NTU) and Chii-Dong Chen (Institute of Physics, Academia Sinica), we have focused on exploring the capabilities of silicon nanowire field-effect transistors (SiNW-FETs) in detecting molecules released from neurons (Figure 1). The SiNW-FET-based biosensor is a reliable, sensitive, label-free, and real-time tool that has been widely applied to analyze the presence of biological molecules. Through modification of the surface with appropriate receptors, the device is able to recognize specific target molecules with a sensitivity at the pM level. Compared to the production and manipulation required for other similar techniques, such as Biacore, which requires an expensive optical instrument, the low-cost production and ease of manipulation of this device makes this technique ideal for biological research. Furthermore, the SiNW-FET could be applied to detect cell activities in real time. We have obtained a patent for applying this design to study biomolecular interactions (Reusable Nanowire Field-Effect Transistor System for Detecting Biomolecular Interactions, US Patent number 8420328; 2013/4/16).
To detect various molecules released from cultured cells, we modified the surface of SiNW-FET with oligonucleotide aptamers. Aptamers can form a structure to bind target molecules specifically. We have verified that dopamine and neuropeptide Y are released differentially from pheochromocytoma (PC12) cells stimulated with different concentrations of ATP (Banerjee et al., 2016); in addition, we have shown that the efflux of K+ from excited neurons elevates the local extracellular K+ concentration (Anand et al., 2017).
In this report, (Anand et al., 2018) (Figure 2), we first chemically modified the Zn2+-sensitive fluorophore, FluoZin-3, so it can anchor onto the SiNW-FET (FZ-3/SiNW-FET). This FZ-3/SiNW-FET has good specificity against Zn2+ (dissociation constant, Kd, ~12 nM) over other divalent cations. To quantify the Zn2+ released from excited neurons in real time, we placed a coverslip seeded with cultured embryonic cortical neurons atop an FZ-3/SiNW-FET, and the [Zn2+]e increased to ~110 nM upon stimulation with α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA). Blockers against AMPA receptors (AMPARs) and exocytosis greatly suppressed the AMPA-induced elevation in [Zn2+]e. In addition, a SiNW-FET modified with Aβ (Aβ/SiNW-FET) bound Zn2+ with a Kd of ~633 nM. Placing neurons atop the Aβ/SiNW-FET and stimulation with AMPA induced a significant change in conductance. These results reveal that neurons release Zn2+ from synaptic vesicles upon stimulation and that the free [Zn2+]e surrounding the excited neurons is high enough to bind Aβ.
[Zn2+]e homeostasis is important for the health of the brain. Understanding the [Zn2+]e surrounding neurons is critical in evaluating the physiological functions of Zn2+. Our findings show that the [Zn2+]e surrounding overexcited neurons is ~115 nM, which is already sufficient to support the formation of the Zn2+-Aβ complex. Considering that Aβ fibrillary aggregates are a pathogenic factor for Alzheimer’s disease, the Zn2+ coreleased with glutamate at the axonal terminals must be under strict regulation to avoid the formation of Aβ-Zn2+ complexes. In addition, this [Zn2+]e level is able to bind to NMDA receptors, which have a high affinity (~ nM level) for Zn2+, to regulate neurotransmission. In conclusion, our SiNW-FET system is sufficiently sensitive to characterize physiological [Zn2+]e in real time and examine how Zn2+ homeostasis modulates neuronal activities.
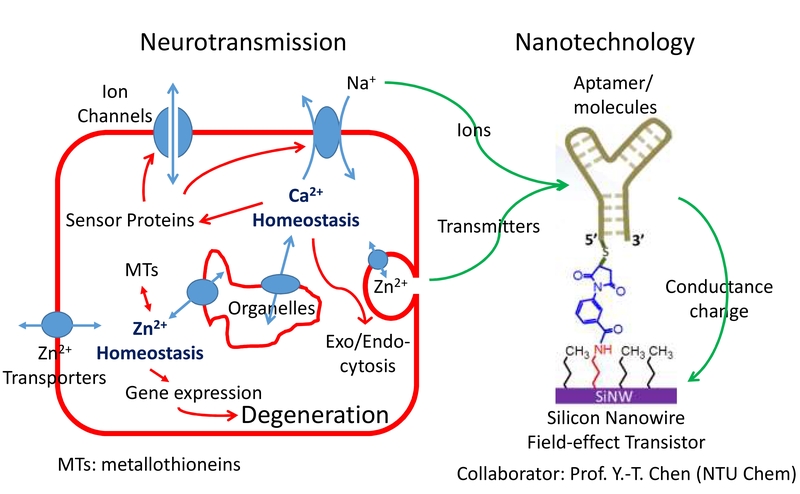
Figure 1. Applying the SiNW-FET technique to study molecules released from cells. Biological approaches reveal that both intracellular Ca2+ and Zn2+ signaling pathways share similar mechanisms to modulate cell activities. Various transporters or channels regulate the fluxes of ions across those membrane systems; ion-binding proteins in the cytosol activate signaling pathways and regulate gene expression. To detect the few molecules released from neurons via the transporters/channels or synaptic vesicles, we modified the SiNW-FET with different aptamers or molecules as the receptors. The binding of molecules to the receptors changes the field potential surrounding the nanowire, resulting in changes in conductance.
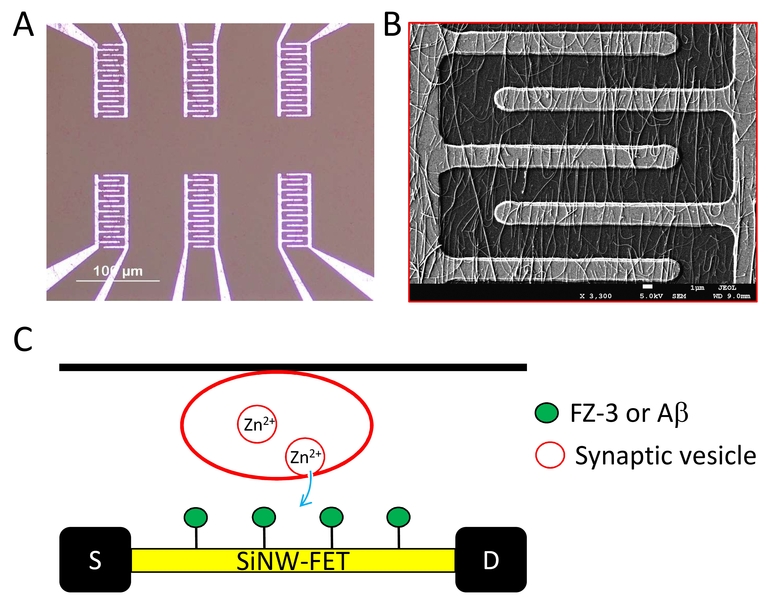
Figure 2. The SiNW-FET device. A. An image of the device that contains 6 pairs of comb-like circuits. B. A scanning electron microscopy images shows part of the circuit covered with silicon nanowires. C. Illustration of the experimental design. Cells grown on coverslips are placed atop the device with cells facing the circuit.
1. Anand, A., Chi, C., Banerjee, S., Chou, M., Tseng, F., Pan, C., & Chen, Y. (2018). The Extracellular Zn2 Concentration Surrounding Excited Neurons is High Enough to Bind Amyloid-β Revealed by a Nanowire Transistor. Small,14(24), E1704439.
2. Anand, A., Liu, C., Chou, A., Hsu, W., Ulaganathan, R. K., Lin, Y., . . . Chen, Y. (2017). Detection of K+ Efflux from Stimulated Cortical Neurons by an Aptamer-Modified Silicon Nanowire Field-Effect Transistor. ACS Sens.,2(1), 69-79.
3. Banerjee, S., Hsieh, Y., Liu, C., Yeh, N., Hung, H., Lai, Y., . . . Pan, C. (2016). Differential Releases of Dopamine and Neuropeptide Y from Histamine-Stimulated PC12 Cells Detected by an Aptamer-Modified Nanowire Transistor. Small,12(40), 5524-5529.
4. Hung, H., Kao, L., Liu, P., Huang, C., Yang, D., & Pan, C. (2017). Dopamine elevates intracellular zinc concentration in cultured rat embryonic cortical neurons through the cAMP-nitric oxide signaling cascade. Mol Cell Neurosci.,82, 35-45.
Chien-Yuan Pan
Professor, Department of Life Science
cypan@ntu.edu.tw