How cells maintain their genome integrity is our long-term interest. The genome is constantly exposed to various endogenous and exogenous insults, ranging from endogenous replication stress to exogenous ultraviolet light and carcinogens. These insults cause a variety of DNA damage. DNA double-strand breaks (DSBs) are the most lethal chromosomal lesion if not repaired properly. DSBs trigger genomic instability and halt DNA replication. Cells have developed evolutionarily conservative mechanisms to repair DSBs and maintain genome stability. Dysregulation of DSB repair pathways causes cell death or diseases such as cancers.
Non-homologous end joining (NHEJ) and homologous recombination (HR) are the two major DSB repair pathways. NHEJ is an error-prone repair pathway that simply promotes religation of two broken ends. In marked contrast, the homologous recombination-mediated repair pathway is a precise repair mechanism. Notably, recombination machinery is also a prerequisite for stabilizing and reinitiating stalled/collapsed replication forks during replication stress. Thus, dysfunction in HR leads to chromosome fragility and cancer susceptibility.
Mechanistically, HR induced by DSBs is catalyzed by RAD51 recombinase. RAD51 polymerizes ssDNA generated from DSB sites to form a helical filament known as the presynaptic filament and then catalyzes the homology search and DNA strand exchange reaction (Figure 1). During HR, RAD51 activity is tightly regulated by several associated partners, including BRCA1/2, PALB2, RAD51 paralogs, RAD51AP1, and the SWI5-SFR1 complex. These interactions raise intriguing questions regarding how these accessory factors influence RAD51 activity mechanistically and how they coordinate with each other. My laboratory is dedicated to addressing this issue using biochemistry, biophysics and cell-based approaches.
Our research work has made a significant contribution to the understanding of the mechanism by which the mammalian SWI5-SFR1 protein complex stimulates RAD51-mediated DNA repair. Genetic analyses have provided evidence that SWI5 and SFR1 function as a complex to facilitate RAD51-dependent recombination repair. This repair mechanism is evolutionally conserved from yeast to mice to humans. Consistent with genetic findings, our biochemical and biophysical analyses have demonstrated that the heterodimeric SWI5-SFR1 complex physically interacts with RAD51 and enhances the RAD51-mediated strand exchange reaction (1). Importantly, we have provided evidence that the enhancement of RAD51 activity stems from a dual function of the SWI5-SFR1 complex, namely, stabilizing the presynaptic filament and enhancing the release of ADP from the filament to maintain the presynaptic filament in its active, ATP-bound form (2). Most importantly, we provided evidence that SWI5-SFR1 interacts with the oligomeric, but not monomeric, form of RAD51. We further identified a mutant variant of SWI5 that forms a complex with SFR1 but abolishes the interaction with RAD51. Our biochemical analyses documented that this mutant variant lacks the abilities to stabilize the presynaptic filament, facilitate ATPase activity by RAD51, and stimulate RAD51-mediated DNA strand exchange. Our results demonstrated that the stimulatory effect of SWI5-SFR1 on RAD51 activity stems from the physical protein-protein interaction (3; Figure 2).
Most importantly, knowledge of recombination-mediated DNA repair obtained from bench work has been translated into clinical use for the prevention and treatment of various cancers that arise due to recombination repair deficiency. Hereditary genetic mutations in recombination repair genes, such as BRCA1, are associated with a significantly high incidence of breast and ovarian cancers. Angelina Jolie, the famous American actress who harbors a hereditary BRCA1 mutation, chose prophylactic surgery for cancer prevention. In addition to cancer prevention, cancer cells with recombination repair deficiency are sensitive to PARP inhibitors, such as olaparib (Figure 3). Thus, diagnosis of recombination repair capacity in cancers will assist in the selection of a specific drug to achieve personalized medicine. In summary, our research on repair mechanisms has great application value for precision medicine.
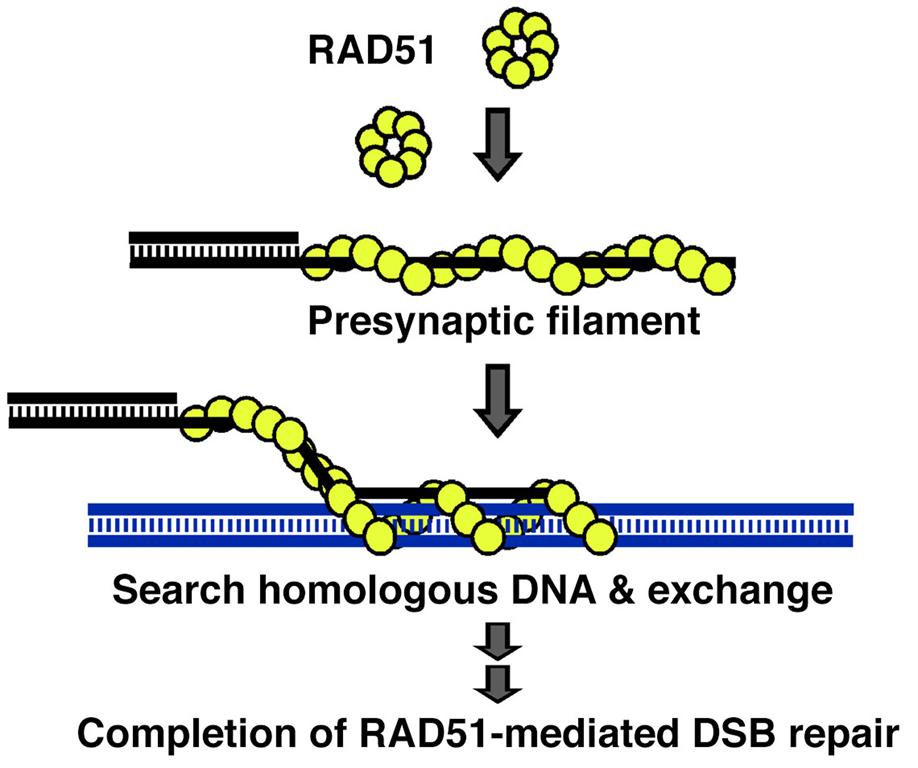
Figure 1. RAD51-mediated homologous recombination repair
The DNA double-strand break (DSB) is resected to generate 3’ ssDNA overhangs. Invasion of a homologous DNA molecule by a RAD51 filament yields a DNA exchange reaction. Following DNA synthesis and DNA ligation, the DSBs are repaired.
Figure 2. Model depicting the mechanisms of SWI5-SFR1 in RAD51-mediated DNA repair
Our study represents a significant conceptual advancement in understanding the mechanistic underpinnings of RAD51-SWI5-SFR1-dependent chromosome damage repair.
Figure 3. Homologous recombination (HR) and personalized medicine
Cancer cells harboring genetic mutations in HR repair genes, including BRCA1 and BRCA2, are sensitive to olaparib. Thus, determining the HR capacity of cancers will have great application value for precision medicine.
References
1. Shang-Pu Tsai, Guan-Chin Su, Sheng-Wei Lin, Chan-I. Chung, Xiaoyu Xue, Myun Hwa Dunlop, Yufuko Akamatsu, Maria Jasin, Patrick Sung, and Peter Chi (2012). Rad51 presynaptic filament stabilization function of the mouse Swi5–Sfr1 heterodimeric complex. Nucleic Acids Research, 40(14), 6558-6569. DOI:10.1093/nar/gks305.
2. Guan-Chin Su, Chan-I Chung, Chia-Yu Liao, Sheng-Wei Lin, Cheng-Ting Tsai, Tao Huang, Hung-Wen Li, and Peter Chi (2014). Enhancement of ADP release from the RAD51 presynaptic filament by the SWI5-SFR1 complex. Nucleic Acids Research, 42(1), 349-358. DOI:10.1093/nar/gkt879.
3. Guan-Chin Su, Hsin-Yi Yeh, Sheng-Wei Lin, Chan-I Chung, Yu-Shan Huang, Yi-Chung Liu, Ping-Chiang Lyu, and Peter Chi (2016) Role of the RAD51-SWI5-SFR1 ensemble in homologous recombination. Nucleic Acids Research, 44(13), 6242-6251. DOI:10.1093/nar/gkw375.
Associate Professor Hung-Yuan (Peter) Chi
Institute of Biochemical Sciences
peterhchi@ntu.edu.tw