Reaction pathways and intermediate products in CO oxidation and CO2 reduction
The realization of a carbon-neutral energy cycle is one of the most imminent issues of our time for human society and civilization. Finding efficient pathways for the reduction of carbon dioxide (CO2) and the oxidation of various types of hydrocarbons (carbon monoxide (CO) is the simplest example) is therefore relevant not only to a wide range of social interests but also represents a fundamental problem in chemistry and material science. Various experimental and computational groups have taken on these challenges. If these groups were to collaborate with each other, they could achieve results beyond the normal scale of discovery. The group led by Dr. Michitoshi Hayashi at the Center for Condensed Matter Sciences, National Taiwan University, for example, was given the opportunity to collaborate with Argonne National Laboratory via the Dragon Gate Project with the goal of establishing size-selected subnanometer metal clusters catalysts, among which subnanometer gold and silver clusters show selective reactions, such as CO oxidation, acetylene oxidation, and NOx reduction. The successful design and realization of subnanometer metal cluster catalysis may, however, face several challenges in (i) the production of the selective size of clusters, (ii) the elucidation of metal-support interaction effects, and (iii) the characterization of clusters under reaction conditions.
Although X-ray absorption near-edge spectroscopy (XANES) and extended X-ray absorption fine structure spectroscopy (EXAFS) measurements can reveal changes in the oxidation state of the catalyst during oxidation-reduction (redox) reactions, specific details regarding the clusters cannot be deduced, which leads to a gap in both what is observed in experiments and what may occur in the sample during measurement. First principles theoretical simulations for predicting both the geometries of the relevant components of the sample and their spectroscopic characteristics may shed light on atomic-scale behaviors and provide in-depth insights (or chemical reaction mechanisms) into what is most likely occurring during the experiments and help experimentalists bridge the knowledge gap.
Taking Ag4 and Ag16 clusters on a TiO2 surface for CO oxidation as an example, the simulation results predict the most and second-most energetically likely sizes of Ag clusters adsorbed on the TiO2 surface. From these geometries, the simulation further identifies several important intermediate products interacting with the Ag4 and Ag16 clusters supported by the TiO2 surface and thus provides a possible CO oxidation reaction pathway for each cluster case as shown in Fig. 1. EXAFS functions for Ag4 and Ag16 clusters in a CO gas environment are calculated and compared with the experimentally observed data (Fig. 2), and the results confirm the validity of the simulated geometries and thus the predicted specific chemical activities of Ag4 and Ag16 clusters supported on TiO2.
Several other studies of energy-related topics, including the elucidation of a reaction mechanism for CO2 reduction on transition metal sulfur materials or the design of an efficient electrocatalyst for the hydrogen evolution reaction on transition metal alloy phosphides, are being pursued in a similar fashion: by determining the geometries of relevant species or important components of the sample, by predicting the reaction pathways and by confirming the predictions using simulations of the spectroscopic properties of these geometries.
|
Figure 1. Geometries and relative energies of (a) molecular O2 and (b) dissociative 2O adsorbed on an Ag4 cluster and (c) molecular O2 and (d) dissociative 2O adsorbed on an Ag16 cluster. Adapted with permission from Ref. [1], Copyright (2017) American Chemical Society. |
|
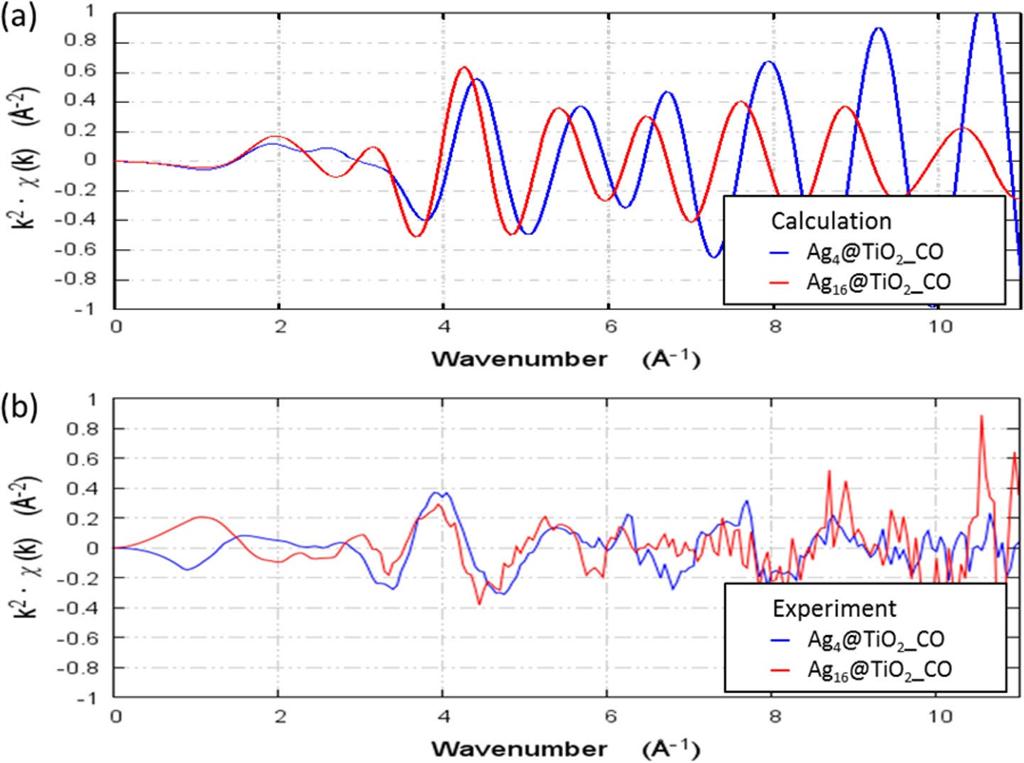
Figure 2. (a) Theoretical and (b) experimental EXAFS obtained for supported Ag4 and Ag16 clusters in a CO gas environment. Adapted with permission from Ref. [1], Copyright (2017) American Chemical Society.
|
Reference
Po-Tuan Chen, Eric C. Tyo, Michitoshi Hayashi, Michael J. Pellin, Olga Safonova, Maarten Nachtegaal, Jeroen A. van Bokhoven, Stefan Vajda, and Peter Zapol. (2017).Size-Selective Reactivity of Subnanometer Ag4 and Ag16 Clusters on a TiO2 Surface. The Journal of Physical Chemistry C, 121 (12), 6614-6625. Published online March 8, 2017. DOI: 10.1021/acs.jpcc.6b11375.
Dr. Michitoshi Hayashi
Research Fellow
Center for Condensed Matter Sciences
atmyh@ntu.edu.tw